Cultural Beliefs and Stakeholder Affiliation Influence Attitudes Towards Responsible Research and Innovation | Frontiers in Political Science
Jennifer Kuzma, June 24, 2021 | Biotech developers are concerned about the future of gene editing having experienced the contentious history of first-generation GM foods. They have also expressed desires to do better with public engagement in gene-editing innovation. The framework of Responsible Research and Innovation (RRI) may provide a way forward to act on their desires for greater public legitimacy....
First GMO Mosquitoes to Be Released In the Florida Keys | Undark
The EPA approved Oxitec's mosquitoes for release this spring. Some scientists and locals want to halt the deployment....Continue reading "First GMO Mosquitoes to Be Released In the Florida Keys | Undark"

Scientists Set a Path for Field Trials of Gene Drive Organisms | Science
Press Release, December 17, 2020 | As genetically engineered organisms ramp up, a multidisciplinary coalition offers a framework for ethical, socially engaged and transparent field practices...Continue reading "Scientists Set a Path for Field Trials of Gene Drive Organisms | Science"

Researchers Recommend More Transparency for Gene-Edited Crops | Science
Press Release, November 19, 2020 | New government regulations for biotechnology will create gaps in oversight of gene-edited crops and the provision of information to consumers....Continue reading "Researchers Recommend More Transparency for Gene-Edited Crops | Science"

Responsible Innovation in Biotechnology: Stakeholder attitudes and implications for research policy | Elementa
Jennifer Kuzma, September 1, 2020 | This article explores attitudes of stakeholders involved in biotechnology towards the Responsible Innovation (RI) framework. ...
Blog: We must do better…
Todd Kuiken, June 11, 2020 | The following reflection was part of a special GES colloquium held on June 5, 2020, discussing the new USDA regulations on GM crops. Which was held in the midst of national protests against police brutality. They are my personal reflections in support of #blacklivesmatter and the systemic racism and inequalities seen throughout our institutions....
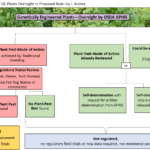
Perspectives on the new USDA regulation on GM crops | GES Colloquium
GES faculty will review how the new USDA rule changes regulation of GM crops in comparison to the agency's previous regulatory approach and in light of findings from the 2016 NASEM GM Crops committee. Diverse perspectives on the new USDA rule will be shared followed by Q and A and discussion with the attendees....Continue reading "Perspectives on the new USDA regulation on GM crops | GES Colloquium"

Career Panel in Risk Science | GES Colloquium
Are you interested in pursuing a career in risk science? If so, please join us to hear from 5 panelists who represent a range of careers in various aspects of risk, including: assessment, communication, governance, and management. ...Continue reading "Career Panel in Risk Science | GES Colloquium"

Lessons Learned for Risk Governance of Synthetic Biology, Nanomaterials, and Other Emerging Technologies in a Post-2020 World
Khara Grieger and Todd Kuiken, Dec. 13, 2019 | On December 9th, a symposium was held at the 2019 Annual Meeting of the Society for Risk Analysis, entitled “Risk Analysis of Engineered Nanomaterials: Where Have We Been, Lessons Learned, and Transfer of Knowledge to Other Emerging Technologies,” as a part of the Advanced Materials and Technologies Specialty Group....
Does the US public support using gene drives to control agricultural pests?
Mike Jones, Sep. 11, 2019 | The development of gene drives is progressing more rapidly than our understanding of public values towards these technologies. Findings from this research can inform responsible innovation in gene drive development and risk assessment....Continue reading "Does the US public support using gene drives to control agricultural pests?"

Cami Ryan on being a social scientist in the Ag industry | GES Colloquium
Cami Ryan joins us from Bayer CropScience and explores the challenges of food, technology, and societies through the lens of a “boundary spanning” personal narrative: a social scientist working in the ag industry....Continue reading "Cami Ryan on being a social scientist in the Ag industry | GES Colloquium"

Jonas Monast – Natural Resources Law as a Model for Biotechnology Governance | GES Colloquium
Jonas Monast joins us from the UNC School of Law to discuss the emerging conflicts between biotechnology governance and natural resources management, and explore how existing natural resources laws can inform biotechnology governance challenges....
Todd Kuiken – Updates and Opportunities in the International Synbio Policy Space | GES Colloquium
GES Colloquium, 9/10/19 | Dr. Todd Kuiken will provide an update on major international treaties and discussions around synthetic biology, and ways for NC State and the broader synbio community to participate in these activities....
USDA to biotech: Call your own compliance
Steven Suppan, July 30, 2019 | The U.S. Department of Agriculture wants agribusiness to sell more genetically engineered (GE) seeds and food products all over the world, as soon as possible. This rule would go beyond already controversial genetically modified organisms (GMOs) to encompass hundreds of new products of new gene and genome editing techniques. The fastest way to do that?...Continue reading "USDA to biotech: Call your own compliance"