
Experts from 14 Nations Discuss Global Gene Drive Project Registry
By Yadira Galindo | UC San Diego Herbert Wertheim School of Public Health and Human Longevity Science led 70 participants from 14 nations, including several GES Center faculty, in a discussion on the ways in which a gene drive project registry could both contribute to and detract from the fair development, testing and use of gene-drive modified organisms...Continue reading "Experts from 14 Nations Discuss Global Gene Drive Project Registry"

NC State part of $26 million grant to study microbiomes
Heidi Reid, September 7, 2022 | NC State is taking part in the National Science Foundation Engineering Research Center for Precision Microbiome Engineering (PreMiEr) to research genetically engineered microbiomes. ...Continue reading "NC State part of $26 million grant to study microbiomes"
Exploring the Social, Ethical Sides of Microbiome Engineering
Nash Dunn, September 7, 2022 | At NSF center, NC State to Lead Research on Societal and Ethical Implications of Emerging Technologies...Continue reading "Exploring the Social, Ethical Sides of Microbiome Engineering"

Researchers Propose New Framework for Regulating Engineered Crops
Mick Kulikoswki, September 1, 2022 | A Policy Forum article published today in Science calls for a new approach to regulating genetically engineered (GE) crops, arguing that current approaches for triggering safety testing vary dramatically among countries and generally lack scientific merit – particularly as advances in crop breeding have blurred the lines between conventional breeding and genetic engineering....Continue reading "Researchers Propose New Framework for Regulating Engineered Crops"

FUN-CROPS
FUN-CROPS About Research scientists at North Carolina State University are exploring ways to harness plant fungal symbionts – specifically foliar fungal endophytes – to improve crop resistance to stressors such as drought, pests, and pathogens....
Cultural Beliefs and Stakeholder Affiliation Influence Attitudes Towards Responsible Research and Innovation | Frontiers in Political Science
Jennifer Kuzma, June 24, 2021 | Biotech developers are concerned about the future of gene editing having experienced the contentious history of first-generation GM foods. They have also expressed desires to do better with public engagement in gene-editing innovation. The framework of Responsible Research and Innovation (RRI) may provide a way forward to act on their desires for greater public legitimacy....
Scientists Set a Path for Field Trials of Gene Drive Organisms | Science
Press Release, December 17, 2020 | As genetically engineered organisms ramp up, a multidisciplinary coalition offers a framework for ethical, socially engaged and transparent field practices...Continue reading "Scientists Set a Path for Field Trials of Gene Drive Organisms | Science"

Researchers Recommend More Transparency for Gene-Edited Crops | Science
Press Release, November 19, 2020 | New government regulations for biotechnology will create gaps in oversight of gene-edited crops and the provision of information to consumers....Continue reading "Researchers Recommend More Transparency for Gene-Edited Crops | Science"

Responsible Innovation in Biotechnology: Stakeholder attitudes and implications for research policy | Elementa
Jennifer Kuzma, September 1, 2020 | This article explores attitudes of stakeholders involved in biotechnology towards the Responsible Innovation (RI) framework. ...
Cami Ryan on being a social scientist in the Ag industry | GES Colloquium
Cami Ryan joins us from Bayer CropScience and explores the challenges of food, technology, and societies through the lens of a “boundary spanning” personal narrative: a social scientist working in the ag industry....Continue reading "Cami Ryan on being a social scientist in the Ag industry | GES Colloquium"

USDA to biotech: Call your own compliance
Steven Suppan, July 30, 2019 | The U.S. Department of Agriculture wants agribusiness to sell more genetically engineered (GE) seeds and food products all over the world, as soon as possible. This rule would go beyond already controversial genetically modified organisms (GMOs) to encompass hundreds of new products of new gene and genome editing techniques. The fastest way to do that?...Continue reading "USDA to biotech: Call your own compliance"
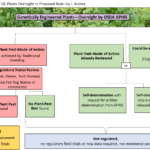
Biotechnology Oversight Gets an Early Make-Over by Trump’s White House and USDA: Part 2 – The USDA-APHIS Rule
Jennifer Kuzma, July 2, 2019 | USDA-APHIS has proposed an oversight process for GE crops that appears to be a significant departure from the current one. This article discusses the features of the proposed new rule, along with its strengths and weaknesses and my recommendations for how it should be amended. ...
Biotechnology Oversight Gets an Early Make-Over by Trump’s White House and USDA: Part 1—The Executive Order
Jennifer Kuzma, June 18, 2019 | Last week, the Trump administration set the tone for its oversight of agricultural biotechnology (ag biotech) through two major actions: 1) Signing the Modernizing the Regulatory Framework for Agricultural Biotechnology Products Executive Order; and 2) Proposing a draft rule on the Movement of Certain Genetically Engineered Organisms (GEOs), changing how USDA reviews GE plants....
Governing evolution – A socioecological comparison of resistance management for Bt crops
Zachary Brown, March 21, 2019 | Cooperative management of pest susceptibility to transgenic Bacillus thuringiensis (Bt) crops is pursued worldwide in a variety of forms and to varying degrees of success depending on context. We examine this context using a comparative socioecological analysis of resistance management in Australia, Brazil, India, and the United States. We find that a shared understanding of resistance risks among government regulators, growers, and other actors is critical for effective governance. ...