On September 12, 2022, President Joe Biden signed Executive Order 14081, Advancing Biotechnology and Biomanufacturing Innovation for a Sustainable, Safe, and Secure American Bioeconomy, intended to “accelerate biotechnology innovation and grow America’s bioeconomy across multiple sectors, including a range of industries, including health, agriculture, and energy.” Below is a set of recent publications from GES Center faculty relating to the content of the EO.
See all GES Center faculty publications here >
| Article | Year | Significance | Keywords | Article Image |
|---|---|---|---|---|
| Furgurson, J., Loschin, N., Butoto, E., Abugu, M., Gillespie, C. J., Brown, R., Ferraro, G., Speicher, N., Stokes, R., Budnick, A., Geist, K., Alirigia, R., Andrews, A., & Mainello, A. (2023). Seizing the policy moment in crop biotech regulation: An interdisciplinary response to the Executive Order on biotechnology. Frontiers in Bioengineering and Biotechnology, 11, 1241537. DOI: 10.3389/fbioe.2023.1241537. PDF. | 2023 | This article critically examines the Biden Administration's Executive Order on Biotechnology and its implications for the regulatory landscape of agricultural biotechnology products. By highlighting historical challenges and the current gaps in the US regulatory system, the authors underscore the urgent need for enhanced transparency and public engagement. Their proposed solutions, including the creation of a shared database ecosystem and reforming public engagement practices, offer a roadmap for building public trust and ensuring responsible biotechnology research and development. | Genetic engineering, Coordinated framework, GMO, Public engagement, Policy | |
| Gould, Fred, et al. "Toward product-based regulation of crops: Current process-based approaches to regulation are no longer fit for purpose." Science 377, 1051 (2022). DOI: 10.1126/science.abo3034 (*Open-access link) | 2022 | A Policy Forum article calling for a new approach to regulating genetically engineered (GE) crops argues that current approaches for triggering safety testing vary dramatically among countries and generally lack scientific merit – particularly as advances in crop breeding have blurred the lines between conventional breeding and genetic engineering. | Product-based Regulation, CRISPR, Bioengineered Crops | 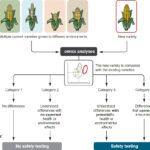 |
| Kuzma, J. Implementing responsible research and innovation: a case study of U.S. biotechnology oversight. Global Public Policy and Governance: 1-19. (2022). doi: 10.1007/s43508-022-00046-x (*Open-access link). PDF | 2022 | This article explores two research questions through a case study of U.S. biotechnology oversight: why visions of Responsible Research and Innovation (RRI) are difficult to implement in governance systems for emerging technologies, and how to get policies and programs to overcome barriers to RRI implementation on the national policy agenda. | Responsible Research and Innovation (RRI), Governance Systems, Emerging technologies | 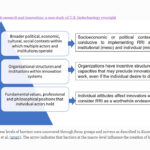 |
| Merck, A. W., Grieger, K. D., Cuchiara, M., & Kuzma, J. (2022). What Role Does Regulation Play in Responsible Innovation of Nanotechnology in Food and Agriculture? Insights and Framings from U.S. Stakeholders. Bulletin of Science, Technology & Society. doi: 10.1177/02704676221102066. PDF* [*PDF requires Unity ID login] | 2022 | Historically, market regulation has played an important role in shaping the trajectory of scientific and technological innovation in food and agriculture. However, regulators’ traditional focus on safety and efficacy may be insufficient to address more complex ethical, legal, and social implications (ELSI) of novel products, such as the use of nanotechnology and nanomaterials in food and agriculture (nano-agrifoods). | Responsible Research and Innovation (RRI), Stakeholder Engagement, Nanotechnology, Food and Agriculture, Regulation | 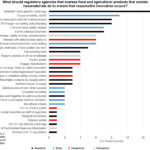 |
| Kuzma, J. (2022). Governance of Gene-edited Plants: Insights from the History of Biotechnology Oversight and Policy Process Theory. Science, Technology, & Human Values. doi: 10.1177/01622439221108225. PDF | 2022 | The history of US biotechnology oversight for genetically modified plants is analyzed in the context of policy process theories to derive insights for contemporary governance of gene-edited plants. | Gene Editing, Policy Process Theory, Biotechnology, Regulation, Governance, GMOs | 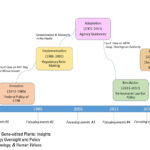 |
| Nelson, K.P., Parton, L.C., Brown, Z.S. Biofuels policy and innovation impacts: Evidence from biofuels and agricultural patent indicators. Energy Policy 162 (2022) 112767. doi: 10.1016/j.enpol.2021.112767. PDF | 2022 | In the early 2000s, governments implemented policies stimulating the use of ethanol and biodiesel to reduce carbon emissions and encourage domestic energy production. We test the impact of blend mandates and other biofuels policies on innovation using measures of patenting activity that correspond with research effort and research output. | Biofuels, Patenting, Biotechnology, Bayesian Model Averaging | 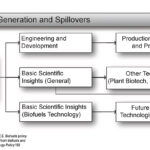 |
| George D.R., Hornstein E.D., Clower C.A., Coomber A.L., Dilliard D., Mugwanya N., Pezzini D.T., and Rozowski C. Lessons for a SECURE Future: Evaluating Diversity in Crop Biotechnology Across Regulatory Regimes. Front. Bioeng. Biotechnol., 02 May 2022. doi: 10.3389/fbioe.2022.886765. PDF. | 2022 | "In this article, we draw upon 30 years of regulatory submission data to 1) understand historical diversification trends across the landscape and history of past crop biotechnology regulatory pathways and 2) forecast how the new SECURE regulations might affect future diversification trends." | Crop Biotechnology, SECURE Rule, Regulation, Diversity trends, Innovation, United States | 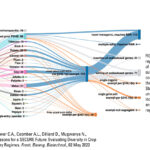 |
| Barnhill-Dilling, S. K., Kokotovich, A., and Delborne, J. A., “The Decision Phases Framework for Public Engagement: Engaging Stakeholders about Gene Editing in the Wild,” in Gene Editing in the Wild: Shaping Decisions through Broad Public Deliberation, ed. Michael K. Gusmano et al., special report, Hastings Center Report 51, no. S2 (2021): S48– S61. DOI: 10.1002/hast.1320 PDF | 2021 | This article explores how public engagement can contribute to the governance of emerging biotechnologies at many points of the technology lifecycle, not just at the point when regulatory agencies issue a permit or not. | Stakeholder Engagement, Environmental Biotechnologies, Gene Editing |  |
| Barnhill-Dilling, SK and Delborne, JA. Whose intentions? What consequences? Interrogating “Intended Consequences” for conservation with environmental biotechnology. Conservation Science and Practice 2021; e406. doi: 10.1111/csp2.406. PDF | 2021 | This article argues that if we seek to emphasize Intended Consequences to embolden innovative conservation efforts, we must simultaneously query whose intentions are included and what consequences are considered to ensure that environmental goals are accompanied by the goals of responsibility, democracy, and justice. | Biotechnology, Conservation, Responsible Research and Innovation (RRI) |  |
| Jaffe G. and Kuzma J. "New Bioengineered (aka GM) Food Disclosure Law: Useful Information or Consumer Confusion?" Food and Drug Law Institute. https://www.fdli.org. Accessed 28 April 2021. PDF | 2021 | This article reviews the labeling standards in the U.S. for bioengineered foods and discusses the strengths and weaknesses. | Bioengineered Food, Regulation, Governance, Transparency, Labeling |  |
| Kuiken T. and Kuzma J. July 2021. Genome Editing in Latin America: Regional Regulatory Overview/Edición génica aplicada a la agricultura: Resumen del marco regulatorio regional. Inter-American Development Bank. DOI: 10.18235/0003410. English version/Versión en español | 2021 | Many countries in the LAC region have established genome editing specific governance systems while others have not specifically implemented genome editing specific governance systems and appear to include them in their current biosafety frameworks. While much of the LAC region appears to be coalescing around a similar interpretation of how genome editing will be governed, it is not yet clear if or how international treaties governing these tools (e.g., Cartagena Protocol on Biosafety to the Convention on Biological Diversity) will ultimately decide. | Regulation, Governance, CRISPR, Latin America, Gene Editing, Inter-American Development Bank |  |
| Kuiken T, Barrangou R, Grieger K. (Broken) Promises of Sustainable Food and Agriculture through New Biotechnologies: The CRISPR Case. CRISPR J. 2021 Feb 10:1-7. doi: 10.1089/crispr.2020.0098. PDF | 2021 | "The CRISPR community therefore must actively engage with these international deliberations, society, and national governance systems that have promised to build better agricultural systems and provide better food products to achieve equitable outcomes while protecting the environment. Without this active engagement, the promises discussed in this paper are sure to be broken." | CRISPR, Stakeholder Engagement, Responsible Research and Innovation (RRI) |  |
| Kuzma, Jennifer, “Deficits of Public Deliberation in U.S. Oversight for Gene Edited Organisms,” in Gene Editing in the Wild: Shaping Decisions through Broad Public Deliberation, ed. Michael K. Gusmano et al., special report, Hastings Center Report 51, no. S2 (2021): S25– S33. DOI: 10.1002/hast.1317 PDF | 2021 | This article analyzes the policy process for emerging biotech products like gene editing and gene drives and the lack of opportunities for public input and deliberation. It proposes ideas for improving deliberation based on the novelty of the product and through a coordinating body like OSTP. | Gene Editing, Public Engagement, Deliberation, Regulation, Governance, Risk Analysis |  |
| Delborne J.A., Kokotovich A.E., and Lunshof J.E. (2020) Social license and synthetic biology: the trouble with mining terms. Journal of Responsible Innovation. doi: 10.1080/23299460.2020.1738023. Published: 06 April 2020. Download PDF | 2020 | After reviewing social license to operate (SLO)’s emergence in the resource extraction context and the existing critiques of SLO, we examine its current use in the synthetic biology literature. We argue that an SLO-derived model of engagement is especially inadequate for synthetic biology due to unique challenges posed by synthetic biology and the limited conception of engagement provided by SLO. | Stakeholder engagement, Public Engagement, Responsible Research and Innovation (RRI), Social license to operate, Synthetic Biology |  |
| Kuzma, J. and Grieger, K. 2020. Community-led governance for gene-edited crops. Science, Vol. 370, Issue 6519. doi: 10.1126/science.abd1512 | 2020 | This article analyzes the USDA's secure rule, it's regulatory pathways and discusses where public information may fall short. It proposes a model for certification and reporting of gene edited crops for market use in order to improve consumer transparency. | Responsible Research and Innovation (RRI), Biotechnology, Governance, CRISPR, Gene Editing | 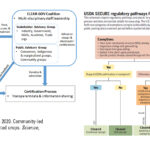 |
| George D.R., Kuiken T., and Delborne J.A.. Articulating ‘free, prior and informed consent’ (FPIC) for engineered gene drives. Proc. Royal Soc. B. Vol. 286, Issue 1917. Published: 18 December 2019. doi: 10.1098/rspb.2019.1484 | 2019 | Recent statements by United Nations bodies point to free, prior and informed consent (FPIC) as a potential requirement in the development of engineered gene drive applications. As a concept developed in the context of protecting Indigenous rights to self-determination in land development scenarios, FPIC would need to be extended to apply to the context of ecological editing. | Community Engagement, Indigenous Peoples, Responsible Research and Innovation (RRI), Convention on Biological Diversity, Public Engagement, Biodiversity |  |
| Jones M.S., Delborne J.A., Elsensohn J, Mitchell P.D., and Brown Z.S. Does the U.S. public support using gene drives in agriculture? And what do they want to know?. Science Advances 11 Sep 2019; Vol. 5, no. 9, eaau8462. doi: 10.1126/sciadv.aau8462. Download PDF | 2019 | This article reports on a nationally representative sample of U.S. adults' attitudes toward using gene drive to manage agricultural pests. When informed about potential risks, benefits, and two previously researched applications, respondents’ support/opposition depends heavily (+22%/−19%) on whether spread of drives can be limited, non-native versus native species are targeted (+12%/−9%), or the drive replaces versus suppresses target species (±2%). | Gene Drives, Agricultural Biotechnology, Public Engagement, Survey, Public Opinion | 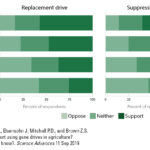 |
| Kuzma J. (2019). Procedurally Robust Risk Assessment Framework for Novel Genetically Engineered Organisms and Gene Drives. Regulation and Governance doi: 10.1111/rego.12245. Download | 2019 | This article critiques the risk analysis process for GM insects as a precursor to gene drives and suggests a new model for risk analysis under the coordinated framework for novel GMOs deployed into complex ecosystems. | Gene Drives, Gene Editing, GMOs, Governance, Risk Analysis | 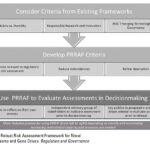 |
| Kuzma, J. Biotechnology Oversight Gets an Early Make-Over by Trump’s White House and USDA: Part 1—The Executive Order. Genetic Engineering and Society Center. June 18, 2019. https://ges.research.ncsu.edu/ | 2019 | Part I of two blogs analyzing the last executive order on the coordinated framework for biotech regulation and what they may mean for responsible oversight of gene edited and other GM products. | Biotechnology, Oversight, Executive Order, SECURE Rule |  |
| Kuzma, J. Biotechnology Oversight Gets an Early Make-Over by Trump’s White House and USDA: Part 2 – The USDA-APHIS Rule. Genetic Engineering and Society Center. JULY 2, 2019. https://ges.research.ncsu.edu/ | 2019 | Part II of two blogs analyzing the last executive order on the coordinated framework for biotech regulation and what they may mean for responsible oversight of gene edited and other GM products. | Biotechnology, Oversight, Executive Order, SECURE Rule, USDA |  |
| Kuzma, Jennifer. Regulating Gene-Edited Crops. Issues in Science and Technology 35, no. 1 (Fall 2018). pp. 80-85. https://issues.org/regulating-gene-edited-crops. Download | 2018 | This article points out four shortcomings in oversight of new gene edited crops that may decrease public confidence and legitimacy given the U.S. emerging approach under the coordinated framework for oversight. | CRISPR, Regulation, Gene Editing, Agriculture |  |
| Kuzma J. (2016). A Missed Opportunity for Biotech Regulation. Science 353: 1211-1213. DOI: 10.1126/Science.Aai7854 | 2016 | This article analyzes the last revisiting of the Coordinated Framework under the Obama administration and suggests improvements for coordination, harmonization and public input into the process. | Biotechnology, Regulation, CFRB | 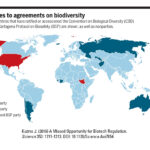 |
| Kuzma, J. (2016). Rebooting the Debate about Genetic Engineering. Nature 531: 165-167. doi: 10.1038/531165A | 2016 | This article argues that product and process distinctions in regulatory approaches to GM crops create artificial distinctions and polarize the discussion. | Biological Techniques, Genetics, Policy, Society |  |
| Kuzma, J. Larson, J. and P. Najmaie. "Evaluating Oversight Systems for Emerging Technologies: A Case Study of Genetically Engineered Organisms," Journal of Law Medicine and Ethics 37 (4): 546-586 (2009). https://doi.org/10.1111/j.1748-720X.2009.00431. | 2009 | Older article from 2009 evaluating the Coordinated framework based on a multi-criteria policy evaluation method. | Oversight, GMOs, CFRB | 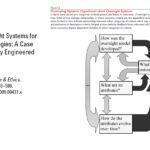 |