Chancellor’s Innovation Fund Catalyzes Six Promising Projects

Research at NC State uncovers potential solutions to some of the world’s grandest challenges. And thanks to the Chancellor’s Innovation Fund (CIF), six new projects will receive support that could bring their research one step closer to market-ready technologies — with broad societal impacts.
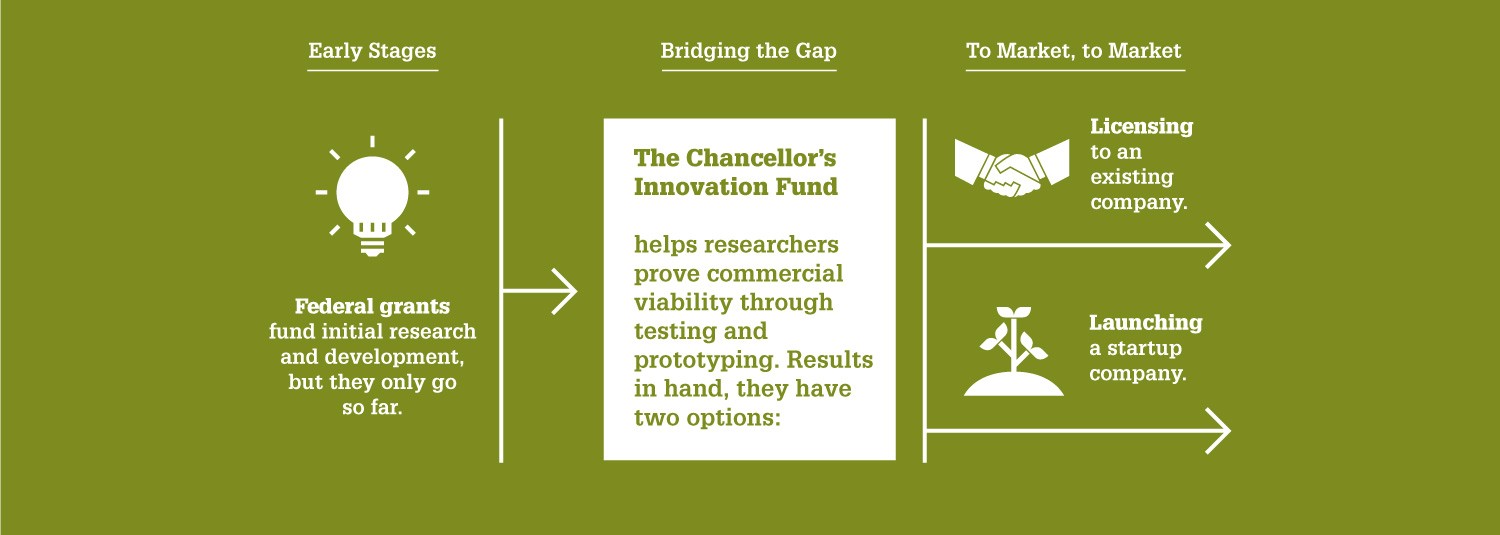
“Since the Chancellor’s Innovation Fund launched over a decade ago, it’s proven to be a highly effective way NC State can help our faculty commercialize their cutting-edge research — and ultimately solve pressing problems,” says Wade Fulghum, assistant vice chancellor of the Office of Research Commercialization. “The goal is to provide the critical funding needed to translate technologies to a point where a startup can be formed for commercialization or a license can be executed with an existing company.”
The CIF, founded in 2010, provides seed funding to a select few NC State research projects each year that have promise for market success. The CIF aims to help these research projects bridge the gap between public and private funding. For every dollar the CIF has awarded, it has generated close to $20 in additional funding or investment.
To date, the CIF has granted nearly $3.7 million to 57 projects — which have attracted over $64 million in follow-on funding. These projects have led to 32 startup companies, 59 license agreements and $1.6 million in licensing revenue.
This year’s CIF-supported projects aim to reshape the manufacture, testing or delivery of products ranging from medicines and immunotherapies to consumer packaging to stealth jets and solar-energy cells.
Slashing the Cost of a Cancer-Killing Cell Therapy
Chimeric Antigen Receptor T (CAR-T) cells are T cells genetically engineered to recognize and destroy cancerous cells. CAR-T cell therapy has revolutionized the treatment of certain types of lymphomas — and it has the potential to revolutionize the treatment of many other cancers, too. Because it takes upwards of a month to manufacture CAR-T cells, though, the treatment costs hundreds of thousands of dollars.
In hopes of dramatically lowering this treatment’s cost, Yevgeny Brudno and his research lab have developed a biomaterial that could shorten the production of CAR-T cells from weeks to mere hours. Their algae-based, dime-sized scaffolds could streamline the production of a variety of genetically engineered cells used in immunotherapies. The ultimate vision is that the scaffolds could be implanted during an out-patient procedure that catalyzes the body to produce its own CAR-T cells naturally.
Brudno, an assistant professor in NC State and UNC-Chapel Hill’s Joint Department of Biomedical Engineering, and Michael Williams, a professor of practice in the same department, will use CIF support to determine the scaffolds’ shelf-life and perform animal trials to look for undesired side effects of the implantable scaffolds.
Finding An Eco-Friendly Alternative to Plastic Packaging

Nearly 8 billion tons of plastic have been produced since 1950 — over half of which has either ended up in landfills or the world’s waterways. Meanwhile, existing biopolymer and fiber-based alternatives require lots of water, energy, and chemicals to produce and therefore cost nearly 10 times as much.
A new, wood-based alternative developed by Lokendra Pal and Lucian Lucia, professors in the Department of Forest Biomaterials, could one day help solve the worsening problem of plastic pollution. They have discovered how to convert leftover sawdust powder and agro-residues into a Styrofoam-like packaging material. The production process is virtually zero-waste and could be scaled to become comparable in cost to plastics. The technology also has other consumer-packaging applications, as well as potential applications in agriculture and construction.
CIF support will be used to conduct pilot and commercial trials of the technology, in advance of testing by potential industry partners.
Building Stealth Jets Better

Stealth fighters and bombers are able to avoid detection largely because of their special skins, which absorb radar. But these skins, made of iron- or carbon-based polymers, are so fragile that the aircraft have to be designed in ways that protect the skin — at the cost of speed, maneuverability and more.
A new, tougher material developed by Cheryl Xu, an associate professor in the Department of Mechanical and Aerospace Engineering, and her research team could allow manufacturers to fundamentally redesign the next generation of stealth aircraft. Xu’s ceramic coating not only absorbs at least 10% more energy from radar than existing polymers, but it’s also able to withstand much harsher conditions — including temperatures as high as 1,800 C. And since it can handle 1,400 C more heat than current state-of-the-art stealth skins, the coating can be applied to an entire aircraft. The coating, applied in a liquid spray that takes two days or less to solidify, could also be used on ships or missiles.
CIF support will be used to further reduce the coating’s thickness, simplify the manufacturing process, and create a larger sample of the material for potential partners and licensees to evaluate.
Making CRISPR Gene Editing Safer and More Effective

CRISPR has quickly become the go-to option for gene editing in agriculture, biotechnology, medicine, research and a variety of other fields. However, one of the main reasons that CRISPR has yet to be widely used to edit human DNA is that the risk of undesired mutations is too high. The root cause of these undesired mutations is that standard CRISPR gene-editing techniques operate by breaking both strands of DNA molecules. While some recently developed CRISPR editing techniques avoid these harmful breaks, the edits they can perform are limited in scope.
Chase Beisel, an associate professor in the Department of Chemical and Biomolecular Engineering, and Ph.D. student Scott Collins have developed a new CRISPR editing technique, with a broad editing scope, that does not cause double-stranded DNA breaks. Beisel and Collins hope their technology could make CRISPR gene therapies safer — and eventually open the door for genetic disease treatments in humans. Their technology is designed to allow for both small- and large-scale edits.
CIF support will be used to further develop the technology, with a priority toward human gene therapies.
Streamlining Quantum Dot Manufacturing with Machine Learning

Colloidal semiconductor nanocrystals, also known as quantum dots, can convert short-wavelength light to almost any color in the visible or infrared spectrum. Quantum dots are used in state-of-the-art LEDs and other optoelectronic devices, as well as next-gen solar cells, energy-efficient greenhouses and more. But because they’re currently made in inefficient batch reactors and also require highly trained manual laborers, quantum dots are not only costly but often vary in quality from batch to batch.
Milad Abolhasani, an associate professor in the Department of Chemical and Biomolecular Engineering, has created an “Artificial Chemist” that utilizes machine learning to modernize the discovery and the manufacturing of quantum dots — which translates to a process that’s nearly 1,000 times faster, at a tenth of the cost. Abolhasani’s autonomous manufacturing process also enables real-time quality control, which could greatly improve the quality of quantum dots manufactured at a large scale.
CIF support will be used to help develop the next generation of the Artificial Chemist technology, in order to demonstrate the self-driven production of high-quality quantum dots at an industrially relevant throughput.
Mimicking the Blood-Brain Barrier More Closely in Preclinical Trials


Most drugs to treat brain and other central nervous system diseases must cross the blood-brain barrier in order to work. That’s why the first step in determining a drug candidate’s viability is often testing the likelihood it will successfully cross the blood-brain barrier. However, current testing methods tend to overestimate that likelihood — which can result in wasted effort and resources when the drug is later found ineffective in clinical trials.
Michael Daniele, an associate professor in the Department of Electrical and Computer Engineering, has developed a microfluidic device that might be able to mimic the blood-brain barrier much more closely than current, 2D testing models can. Daniele’s 3D chip contains two engineered-tissue components — a blood vessel and glial tissue — forming an interface that fits in the palm of your hand. Daniele, who also holds an appointment in NC State and UNC-Chapel Hill’s Joint Department of Biomedical Engineering, says it could also be used to replicate other systems — like the lungs, skin, gut, or even a tumor — simply by swapping out the brain tissue for any type of tissue that fed with capillary blood vessels.
CIF support will be used to validate that the technology can simulate the blood-brain barrier function continuously for multiple days during drug screening.
This post was originally published in NC State News.


