Translational Research Commercialization Support

For researchers interested in developing health-related ideas such as potential therapeutics or devices, as the ideas move closer to the clinic, funding agencies will request more details about the regulatory and commercialization plan as part of the funding proposal. To help you think through these sections, NC State offers researchers a number of services. For example, FastTraCS, a service available through NC State’s partnership with the North Carolina Translational and Clinical Sciences (NC TraCS) Institute, provides no-cost support to help researchers quickly translate discoveries and inventions to market.
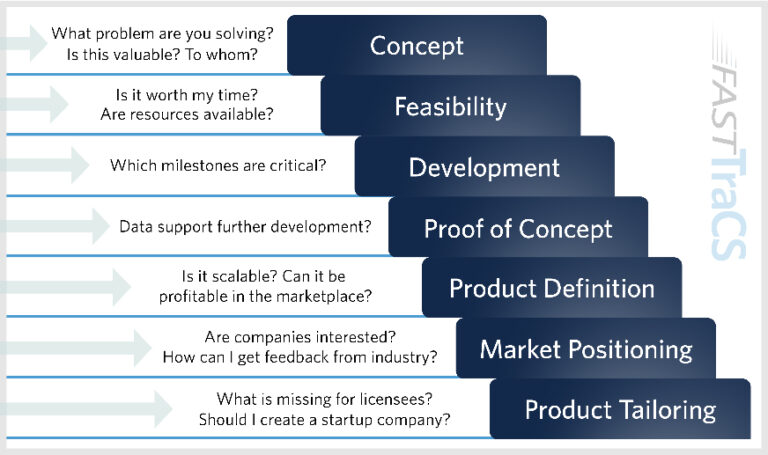
FastTraCS offers consultations for early-stage concepts from existing research and/or clinical observations, recruitment of local industry and technical mentors to help inform development planning and risk assessment, an experienced internal R&D team who identify solvable problems within UNC Health and rapidly develop solutions, referral and group consultations to other resources and partners, and pilot funding to help advance innovative drug discovery, medical device, diagnostic, tools and health/IT ideas. The FastTraCS team supports researchers at every stage of the process — from concept to product tailoring.
Researcher-initiated Product Development
An example of an investigator-initiated project that benefited from substantial support from the FastTraCS team is the Couplet Care Bassinet™. Kristin Tully, PhD approached FastTraCS for assistance because she had an idea to transform maternal-infant care with a new design for in-room bassinets for hospital use, but had no idea how to turn that idea into an FDA-approved product.
The FastTraCS team identified appropriate funding opportunities and connected Tully with Trig, an industrial design firm, and a cohort of engineering students at NC State for the first phase of product development. This phase consisted of design ideation, NC State studio prototypes, refinement of studio prototypes, and design improvements made in conjunction with stakeholder input.
Currently, the Couplet Care team has secured additional funding from the North Carolina Biotechnology Center and the NIH to complete design refinements, produce medical-grade prototypes, conduct safety testing to federal standards, and complete multi-site clinical trials of the bassinet in use in postnatal hospital wards. While delayed a year due to the COVID-19 pandemic, the team hopes to complete this work by May 2021 with an FDA-approved bassinet on the market soon thereafter.
Get in Touch with FastTraCS Today
The FastTraCS program has a strong focus on de-risking technologies for commercial development enabling clinicians and researchers to prioritize studies that advance translational innovations towards a startup company, license to an established firm or directly into clinical practice. To learn more about the FastTraCS program, request a consultation with Andrew Kant, associate director of the FastTraCS service.
One way in which NC TraCS shares information is through a weekly email newsletter that goes out most Wednesdays. Subscribe at tracs.unc.edu/subscribe to get the newsletter delivered to your inbox. It is a great way to learn more about how you can participate in this innovative joint endeavor between NC State and NC TraCS. If you create an account with NC TraCS, you are automatically subscribed to this weekly email.
NC TraCS Institute — Educating, Funding, Connecting, and Supporting Researchers
In 2018, the University of North Carolina at Chapel Hill was awarded a third cycle of its Clinical and Translational Science Awards (CTSA) Program from the National Institutes of Health (NIH). The integrated hub of this CTSA Program at UNC is the North Carolina Translational and Clinical Sciences (NC TraCS) Institute. This award has continued multiple existing collaborations, including with partners North Carolina State University, RTI International, and North Carolina Agricultural and Technical State University.
NC TraCS is a grant-funded institute founded as a service to the research community — guiding researchers through clinical trials and regulatory approval, all the way to implementation in patient care. The mission of NC TraCS is to accelerate discoveries into interventions that improve the health of individuals and the public.
View the NC TraCS Informational Brochure to learn about the resources and services available to research teams.
- Categories: